The Problem Solved by CathClip:
Device Mismanagement Causes Inefficiencies & Safety Issues
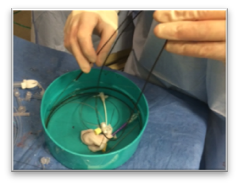
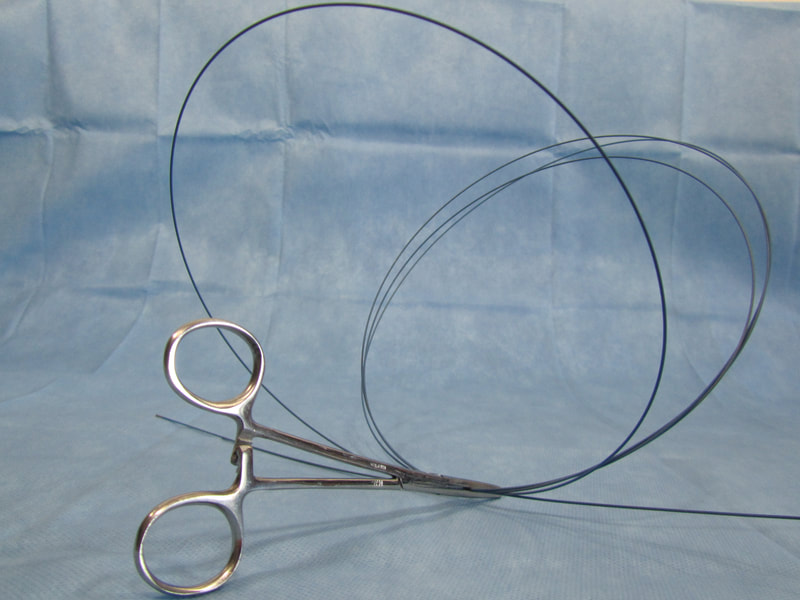
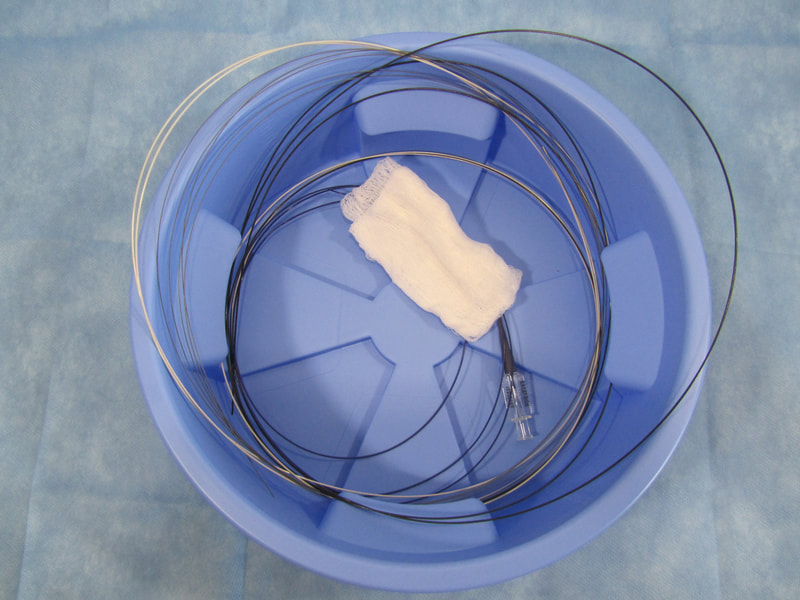
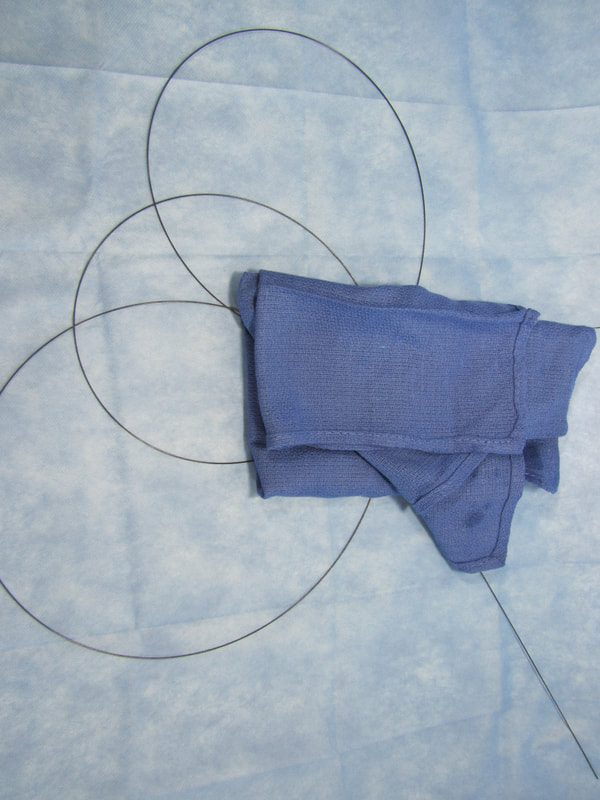
Inefficiencies and complication risks result from the current state of the art in the most cutting-edge medical procedures: between uses, expensive guidewires, catheters, and balloons (flexible elongated medical devices) are managed with makeshift techniques, such as placing them in buckets of saline and wrapping them in gauze and cotton towels. Each device has different attributes, so multiple are used and reused throughout procedures. Without CathClip, these devices are mismanaged, with interventionalists and their assistants using makeshift techniques to handle them, as described below. This mismanagement leads to costly inefficiencies and dangerous complication risks.
- Inefficiencies include extra costs from replacing materials due to damage and/or contamination: over $51/case in all cases and over $231/case in complex cases only, plus wasted procedure time.
- Complication risks include embolization (stroke) from lint, infection from contamination, longer procedure times, and distraction of the procedure team.
- Care team safety risks include unnecessary radiation and COVID exposure.
No single makeshift technique can handle all types of guidewires, catheters, and balloons. There are different types of makeshift techniques because different flexible elongated medical devices must be handled specifically to account for their particular characteristics. For example, (1) stiff wires must be wrapped around themselves to hold in place, (2) hydrophilic wires are held in a bowl since they are too slippery to be held with a wet towel and need to be kept wet, preferably in a saline-filled bowl (if not kept wet, hydrophilic wires and towels or gauze will stick when they become dry), and (3) fragile microwires are held with a wet towel so as to avoid damage.
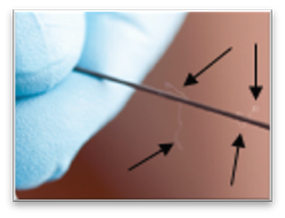
Using makeshift techniques adds costs and safety issues. These techniques are unreliable and difficult to use, taking extra time and often leading to damage to devices, necessitating replacement of devices which would otherwise be reused during the case. Contamination of the devices can also result, and the procedure team is often distracted by device management, with devices sometimes not ready to be used when needed. Further, holding devices with anything with cotton fibers, such as gauze and cotton towels, increases the risk of complications from lint that gets stuck on the devices and then enters the patient's body when the devices are used (embolization/stroke risk).
Without CathClip, inefficiencies are measured at more than an extra $51 per case.
|
Makeshift techniques lead to waste in time and materials. Choosing which makeshift technique to use and getting the chosen technique just right so that it securely holds the flexible elongated medical device without damage can be distracting, time-consuming, and, frankly, quite inefficient. And often, the makeshift technique fails, leading to waste when a flexible elongated medical device touches outside of the sterile field or is otherwise irreparably damaged (kinked) and must be replaced within a single procedure (meaning a duplicate device, for example, a guidewire, catheter, or balloon, must be paid for and time must be taken during the procedure to replace and prepare that new duplicate device for use).
The cost of duplicate/replacement devices alone is estimated to be over $51 per case in all cases and over $231 per case in complex cases only. This figure does not include associated costs of duplicate/replacement devices, such as increased procedure time as replacements must be retrieved from store rooms and prepared for use and extra radiation exposure when damage to devices is noticed only when inside of the patient and not functioning properly. $51 per case includes all cases, both simple and complex. $231 per case includes complex cases only, and studies show that there are more duplicate/replacement devices in complex cases than there are in simple cases. Considering the number of cases at a busy hospital, the extra cost of duplicate devices has been estimated to be at least $1.75 million annually - at one hospital only.
|
Without CathClip, safety issues include risk of foreign material embolization (stroke) from lint, infection, & unnecessary radiation.
|
Wiping and holding devices with materials containing cotton fibers increases the risk of intra-and post-procedure complications (embolization/stroke). See, e.g., Fischi M and Narins CR. Coronary Embolization of a Gauze Fragment: A Cautionary Case Report. Catheterization and Cardiovascular Interventions (2005). Vol. 66, 570-572. Cath labs and hybid ORs are stopping use of items with cotton fibers (for example, gauze and cotton towels) due to the risk of intra-and post-procedure complications associated with embolization of foreign material (lint) stuck on guidewires, catheters, balloons, and similar flexible elongated devices and then introduced into the body during endovascular procedures. New low-lint replacement towels, now being used at many facilities, are lighter in weight and do not hold guidewires like cotton towels do, increasing the problems associated with handling devices with makeshift techniques. Additionally, some have introduced policies of not wiping devices with gauze, switching to more expensive Telfa and other low lint options instead.
|
Additional complications can result from infection risk due to mismanaged devices touching outside of the sterile field. These infections are difficult to track, but often patient care teams use chlorhexidine or Betadine to clean contaminated devices so that they can be reused during a case. Post-endovascular procedure infections are described as a known risk to patients, especially for implanted devices. Any tools which can minimize infection risk, such as CathClip, will help improve clinical outcomes.
Further, procedure teams can be distracted from patient care when they must focus on difficult device management, and often devices are not ready to be used when needed during a procedure. Unnecessary radiation for the patient and care team is another safety issue presented by mismanaged devices, as damage to such devices, such as guidewires and catheters, is often noticed only when not functioning properly inside of the patient, after everyone in the room, including the patient, has been exposed to unnecessary radiation. Increased COVID exposure is another risk of mismanaged devices, as viable, SARS-CoV has been isolated from respiratory, blood, urine, and stool specimens and poorly managed devices can result in blood and contaminated fluids splattering the procedure room and team.
Further, procedure teams can be distracted from patient care when they must focus on difficult device management, and often devices are not ready to be used when needed during a procedure. Unnecessary radiation for the patient and care team is another safety issue presented by mismanaged devices, as damage to such devices, such as guidewires and catheters, is often noticed only when not functioning properly inside of the patient, after everyone in the room, including the patient, has been exposed to unnecessary radiation. Increased COVID exposure is another risk of mismanaged devices, as viable, SARS-CoV has been isolated from respiratory, blood, urine, and stool specimens and poorly managed devices can result in blood and contaminated fluids splattering the procedure room and team.
Summary of Problems Caused by Mismanagement of Devices
Mismanagement of devices leads to a number of problems, including those described below.